将废石墨阳极升级再利用到预硫化催化剂中:实现无阳极电池原型的分离策略
太阳能暖风机通过太阳能电池板加热,无化石燃料消耗。 #生活常识# #环保节能技巧# #太阳能利用#
将废石墨阳极升级再利用到预硫化催化剂中:实现无阳极电池原型的分离策略
Ning Yao, Fu Liu, Ahu Shao, Rongrong Xue, Qiurong Jia, Yuyao Liu, Helin Wang, Xin Wang, Yaxin Zhang, Min Zhang, Zhiqiao Wang, Yunsong Li, Jiawen Tang, Xiaoyu Tang, Yue Ma
引用次数: 0
摘要
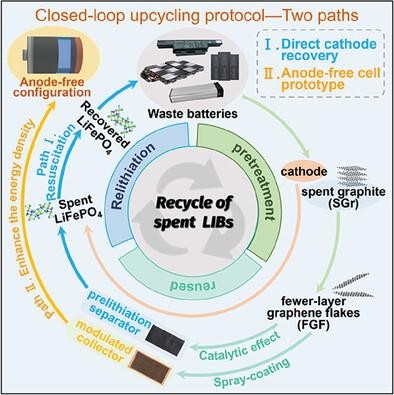
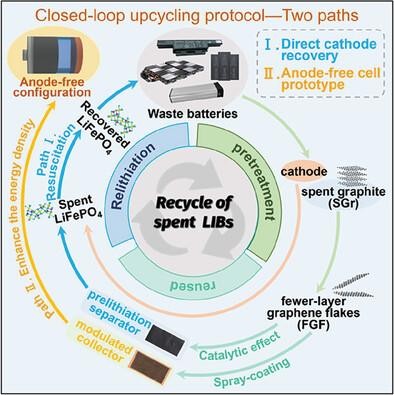
锂离子电池(LIB)的大量生产需要可持续、循环和去碳化的回收策略。虽然人们致力于利用复杂的化学工艺从正极中提取有价值的金属,但直接、高效的正极再生仍是一项技术挑战。更紧迫的是,电池供应链还需要对报废阳极进行增值开发。在此,我们提出了一种 "闭环 "方法,通过对层间距和缺陷浓度的微妙调整,将废石墨循环利用为预锂化催化剂,即少层石墨烯薄片(FGF)。由于催化 FGF 可减轻煅烧过的 Li5FeO4 纳米晶体的脱锂能障,因此,将其浇铸在聚烯烃基底上的复合层可实现定制的预锂化能力(98% 的 Li+ 利用率),用于回收已退役的 LiFePO4。此外,疏水性聚合物改性保证了 Li5FeO4 制剂的耐湿性,符合商业电池制造标准。隔膜策略很好地调节了无阳极袋式电池(LiFePO4||Cu)的界面化学性质,其原型兼顾了稳健的循环性、高达 386.6 Wh kg-1 的能量密度以及 1159.8 W kg-1 的极限功率输出。这项研究不仅实现了石墨升级再循环的可持续供应链,还为无阳极电池原型设计建立了通用、可行的协议。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
Upcycling the Spent Graphite Anode Into the Prelithiation Catalyst: A Separator Strategy Toward Anode-Free Cell Prototyping
The substantial manufacturing of lithium-ion batteries (LIBs) requires sustainable, circular, and decarbonized recycling strategies. While efforts are concentrated on extracting valuable metals from cathodes using intricate chemical process, the direct, efficient cathode regeneration remains a technological challenge. More urgently, the battery supply chain also requires the value-added exploitation of retired anodes. Here, a “closed-loop” approach is proposed to upcycle spent graphite into the prelithiation catalyst, namely the fewer-layer graphene flakes (FGF), upon the exquisite tuning of interlayer spacing and defect concentration. Since the catalytic FGF mitigates the delithiation energy barrier from calcinated Li5FeO4 nanocrystalline, the composite layer of which cast on the polyolefin substrate thus enables a customized prelithiation capability (98% Li+ utilization) for the retired LiFePO4 recovery. Furthermore, the hydrophobic polymeric modification guarantees the moisture tolerance of Li5FeO4 agents, aligning with commercial battery manufacturing standards. The separator strategy well regulates the interfacial chemistry in the anode-free pouch cell (LiFePO4||Cu), the prototype of which balances the robust cyclability, energy density up to 386.6 Wh kg−1 as well as the extreme power output of 1159.8 W kg−1. This study not only fulfills the sustainable supply chain with graphite upcycling, but also establishes a generic, viable protocol for the anode-free cell prototyping.
相关文献
二甲双胍通过HDAC6和FoxO3a转录调控肌肉生长抑制素诱导肌肉萎缩
Min Ju Kang, Ji Wook Moon, Jung Ok Lee, Ji Hae Kim, Eun Jeong Jung, Su Jin Kim, Joo Yeon Oh, Sang Woo Wu, Pu Reum Lee, Sun Hwa Park, Hyeon Soo Kim
具有疾病敏感单倍型的非亲属供体脐带血移植后的1型糖尿病
Kensuke Matsumoto, Taisuke Matsuyama, Ritsu Sumiyoshi, Matsuo Takuji, Tadashi Yamamoto, Ryosuke Shirasaki, Haruko Tashiro
网址:将废石墨阳极升级再利用到预硫化催化剂中:实现无阳极电池原型的分离策略 https://www.yuejiaxmz.com/news/view/856953
相关内容
某工厂采用石墨电极电解Na2CrO4溶液.实现Na2CrO4到 Na2Cr2O7的转化.其装置如图所示.下列说法正确的是( )A.阳极区的PH会不断下降B.溶液中Na+从负极移向正极C.该电解池的两电极材料只能用石墨不能用铁D.阳极区发生的反应是:2H2O二甲基二硫化物(二甲基二硫化物用强极性柱子还是弱极性柱子)
欧阳明高院士最新综述文章:关于锂离子动力电池超级快充的关键问题
利用电解法可将含有Fe.Zn.Ag.Pt等杂质的粗铜提纯.下列叙述正确的是 A.电解时可以精铜为阳极.以CuSO4溶液为电解液B.电解时阴极发生还原反应C.粗铜连接电源负极.其电极反应是Cu
[题目]某新型锂离子可充电电池正极材料是含锂的二氧化钴(LiCoO2).充电时LiCoO2被氧化.Li+迁移并以原子形式嵌入电池负极材料碳(C6)中形成LiC6.下列说法正确的是A. 充电时.锂离子电池的负极发生氧化反应B. 放电时.锂离子电池的正极反应为CoO2+Li++e=LiCoO2C. 放电时.锂离子电池的正极的质量逐渐减小D. 充电时.C6极应与 题目和参考答案——青夏教育精英家教网——
点墨成金 宁波探索石墨烯产业化科创新路径
“碳呼吸电池 是一种新型能源装置.其工作原理如图.下列说法正确的是( )A.该装置是将电能转变为化学能B.正极的电极反应为:C2O42
氧化石墨烯=污水处理【钜大锂电】
九级化下册专题9化与生活单元能源的开发与利用同步练习含解析新版仁爱版.docx
随着化石能源的减少.新能源的开发利用日益迫切.(1)Bunsen热化学循环制氢工艺由下列三个反应组成:SO2(g)+I2(g)+2H2O+H2SO4(l)△H=a kJ?mol

